NEW
Sterilisation: Understanding Load Variables
In Sterilisation, the configuration of the load plays a decisive role in ensuring the effectiveness, safety, and consistency of the process. At Medistri, we support our customers by delivering sterilisation solutions that offer controlled flexibility - always in full compliance with industry and international standards. Managing load variables effectively means not only meeting regulatory requirements but also gaining operational efficiency and efficacy throughout the supply chain.
Solutions for Medical Devices with Integrated Electronics
As the medical industry advances, devices are becoming more compact & connected. Many of today’s innovations include integrated electronics - microchips, sensors, batteries, or embedded software, that allow for monitoring, adjustment, or data transmission. These features improve patient outcomes, but they also create new challenges when it comes to sterilisation.
Sterilisation for Pharmaceuticals
Sterilization is a critical process for ensuring the safety and efficacy of pharmaceutical products, particularly in packaging and injectable solutions. Medistri offers contract sterilisation services that meet rigorous pharmaceutical manufacturing standards.
Steam Sterilization at Medistri
Steam sterilization is an efficient and reliable method for decontaminating medical devices and pharmaceuticals. By exposing products to saturated steam at temperatures between 121°C and 134°C, this process ensures thorough microbial eradication while preserving product integrity. Carefully controlled temperature, pressure, and humidity provide an optimal environment for steam to effectively sterilize every surface. The result is a trusted solution that maintains the functionality of your products while ensuring their safety.
EO Sterilisation & Packaging
The design of a medical device’s packaging plays a critical role in ensuring the product’s success and safety. It should be developed during the early stages of the device’s creation to avoid costly challenges later. Packaging is not just a protective layer; it serves as a sterile barrier that safeguards the medical device from manufacturing to final use.
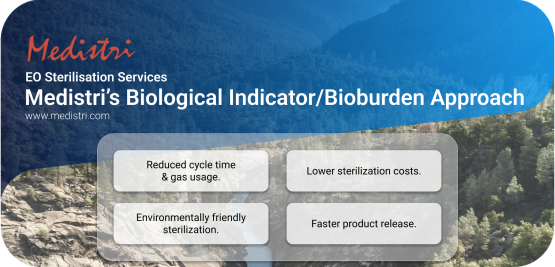
Medistri’s Biological Indicator/Bioburden Approach
At Medistri, we are committed to creating sustainable solutions by focusing on scalable innovation. We aim to drive progress through new technologies, financial structures, and renewable energy deployment. In addition to our gas treatment technology, we have invested resources to offer a smarter alternative to the traditional sterilization methods. One of our key innovations is the "Biological Indicator/Bioburden Approach," a more efficient and environmentally friendly alternative to conventional sterilization.
Medistri's Medical Device Sterilisation
Medical Devices Sterilisation is essential to prevent infections and protect patient health. Adhering to standards like ISO 11135 for EO sterilisation ensures rigorous validation and controls, guaranteeing sterility and maintaining the highest levels of safety and reliability.
Medistri’s Application Areas for Steam Sterilisation
Steam sterilisation is a highly effective and reliable method of decontamination. By exposing products to saturated steam at high temperatures—typically between 121°C and 134°C—steam sterilisation ensures the elimination of harmful microorganisms. The process involves three critical phases: conditioning, exposure, and exhaust, with exposure times ranging from 3 to 15 minutes, depending on the specific product requirements.
Medistri’s Application Areas for EO Sterilisation
EO Sterilisation is particularly beneficial for products that cannot be subjected to high heat or moisture, making it an ideal solution for many modern medical devices and pharmaceutical products. Here’s how it works in practice for different types of products that are in their definitive package.
Sterilisation for Pharmaceutical Vials
A pharmaceutical vial is a small container, typically made of glass or plastic, used to store and protect liquid, powder, or lyophilized (freeze-dried) pharmaceutical substances. These vials must be sterilized and sealed with a rubber stopper or a screw-on cap, often secured by a metal crimp or seal. They are commonly used for injectable medications, vaccines, or other substances requiring precise dosing. Vials ensure the contents are protected from contamination and maintain sterility until use.